In phase 2 of the COVAX Allocation Mechanism, the Independent Allocation Validation Group (IAVG), will transition to a role of strategic advice and monitoring of global and COVAX vaccination equity. The IAVG will perform regular reviews of COVAX’s allocations against principles and objectives of Phase 2 allocation and provide strategic guidance on potential areas for improvement and on policy decisions relating to the allocation of COVID-19 vaccines.
in 2021, WHO, Gavi and CEPI (collectively, the “COVAX Partners”) supported the establishment by WHO of an Independent Allocation of Vaccines Group (“IAVG”) pursuant to the WHO Regulations for Study and Scientific Groups, Collaborating Institutions and Other Mechanisms of Collaboration.The IAVG has played a pivotal role in the allocation of Vaccines under COVAX and to foster the independence of, and provide transparency into, the Vaccine-allocation decision-making under COVAX: Moving to phase 2, the IAVG will pivot its focus in light of the different supply situation and in line with the principles and objectives of Phase 2.
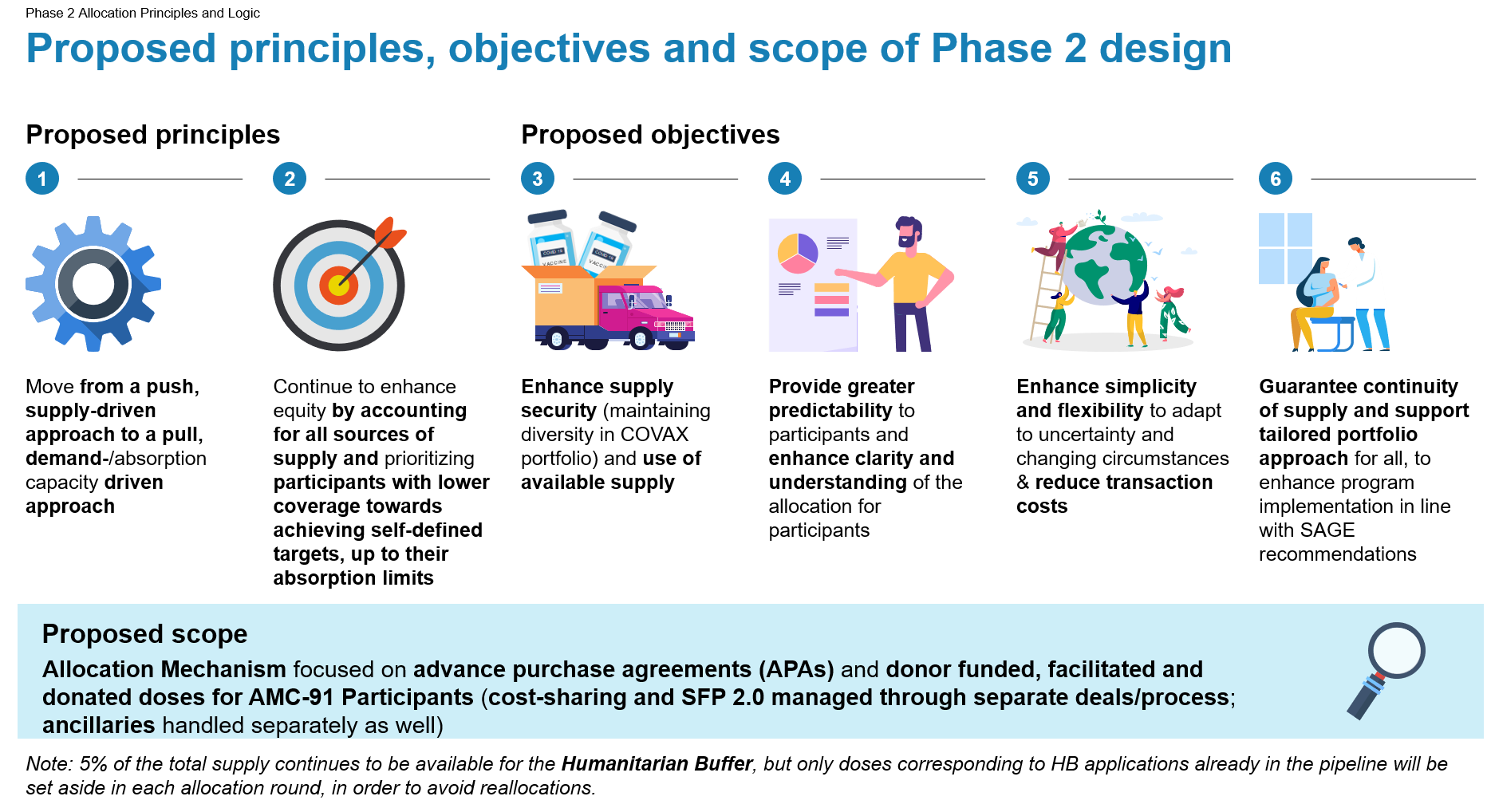
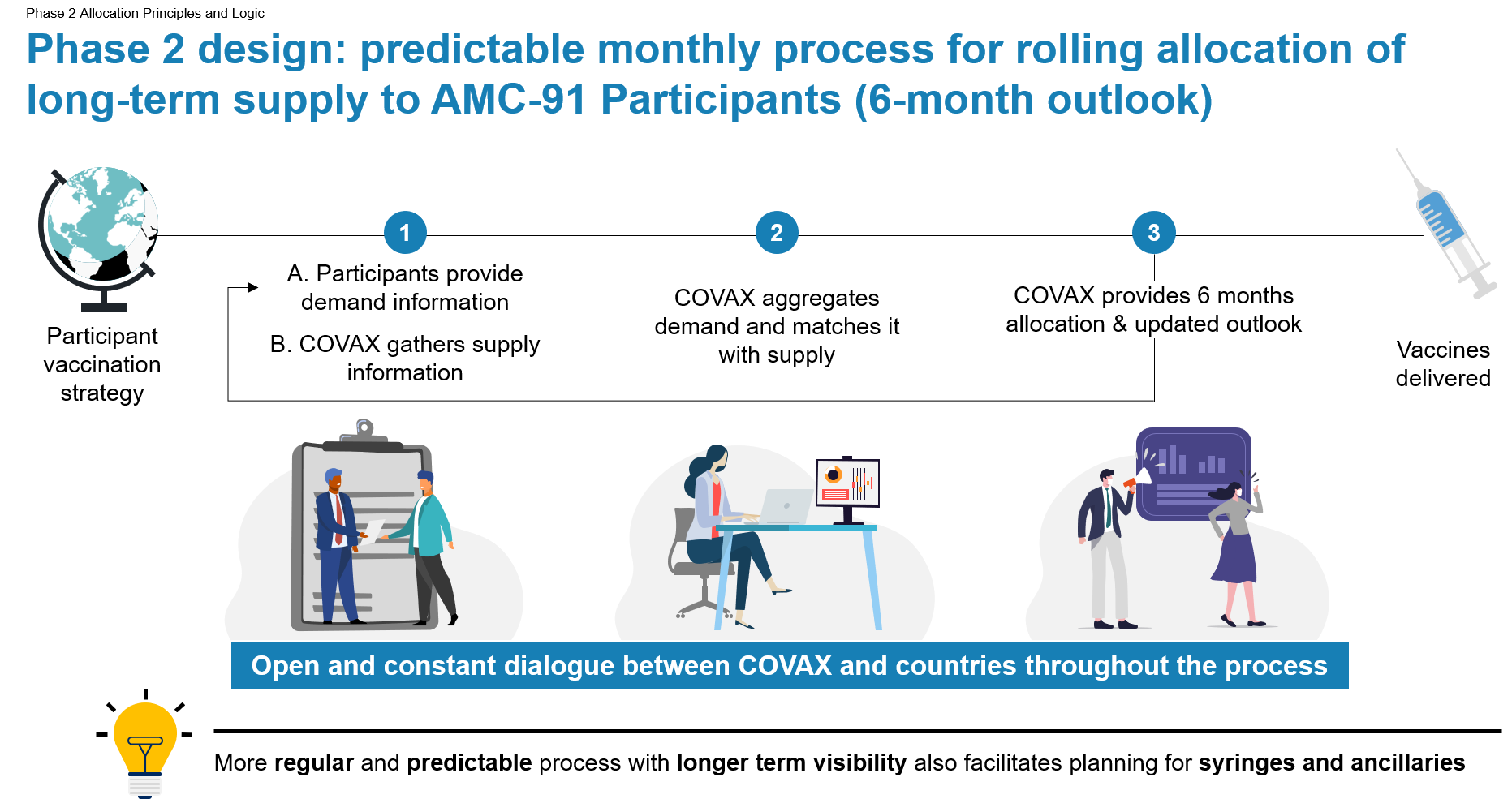
The environment in Phase 2 is expected to be less supply-constrained and the pace at which countries receive vaccines depends on country demand, which drives the allocation processes. In view of this new phase, the Independent Allocation Validation Group (IAVG), will transition to a role of strategic advice and monitoring of global and COVAX vaccination equity. The main tasks of the IAVG will be to perform regular reviews of COVAX’s allocations against principles and objectives of Phase 2 allocation and to provide strategic guidance on potential areas for improvement and on policy decisions relating to the allocation of COVID-19 vaccines. The IAVG might also support additional functions if circumstances change significantly (e.g. approval of certain specific allocations in case of supply constraint).
The IAVG will have the following functions:
- To perform quarterly review of COVAX’s allocations against principles and objectives of Phase 2 Allocation
- To provide strategic guidance on potential areas for improvement and on policy decisions relating to the allocation of COVID 19 vaccines
Documents and reports
Vaccine Allocations Phase 2 – from May 2022
COVAX Allocation Round 16
Vaccine Allocation Decision on the allocation of COVAX Facility secured vaccines: 22 June 2022
Report of the Independent Allocation of Vaccines Group on the allocation of COVAX Facility secured vaccines: 7 June 2022
Vaccine Allocations Phase 2 – from April 2022
COVAX Allocation Round 15


